Current Challenges in CGT Manufacturing
Cell and gene therapies (CGTs) have gained increasing traction over recent years, as they address significant unmet clinical needs such as cancer therapies and rare genetic diseases. The hope for CGTs is that they not only become novel therapies for diseases with little to no treatment options, but also that they become curative, enabling replacement of life-long treatments of chronic conditions.
Nevertheless, despite the promise CGTs have shown to date, the extent of the breakthrough of these technologies is hampered by a number of obstacles, making it difficult for these technologies to truly take off. Technical issues, high costs, increased regulatory constraints… some of the main CGT manufacturing challenges are discussed in this article, along with the potential solutions that could be brought by Focal Molography.
Increasing Regulatory Requirements
As more and more CGTs are being developed and come to market, our knowledge of potential adverse effects and of associated manufacturing parameters is growing. Regulatory bodies such as the FDA are increasingly putting pressure on CGT manufacturers to adhere to new standards. Quality control of critical quality attributes (identity, purity, potency, and safety) is becoming increasingly difficult as the number of parameters to assess augments: particle number, particle concentration, encapsulation efficiency, immunogenicity, polydispersity, aggregation… The assays to be conducted are seemingly endless; and for CGTs in particular, they are expensive. Currently, many of the relevant quality parameters for CGTs can only be accurately assessed using cell-based assays; this implies days to weeks of multiple cell cultures, and all the manual and costly work that comes with it. These assays, although extremely effective at providing the desired read-outs, are poorly scalable and not sustainable long-term should CGTs be upscaled.
Given that regulatory requirements for CGTs are not likely to decrease, but rather will continue to increase as more and more clinical trials are undergone, changing the CGT workflow to adapt to these new and upcoming standards is essential. Ideally, one would circumvent the need for cell-based assays entirely, as they are by far the most cumbersome assays to be performed. Although cell-based assays will likely never fully disappear from the CGT manufacturing workflow, it could be possible to greatly reduce our dependence on them by developing technologies and assays capable to provide sufficiently informative read-out. For instance, by testing how the outcome of cell-based assays correlate with given physio-chemical parameters of a given therapeutic, one could eventually test the parameters that highly correlate with cell-based assay outcome as a proxy for the cell-based assay itself. This would require the collection of large datasets and multiple testing rounds, as well as a technology that lends itself to frequent and rapid testing without compromising on specificity. Focal molography could become one such technology, as its simple use, fast results and highly robust sensing fulfill the desired features.
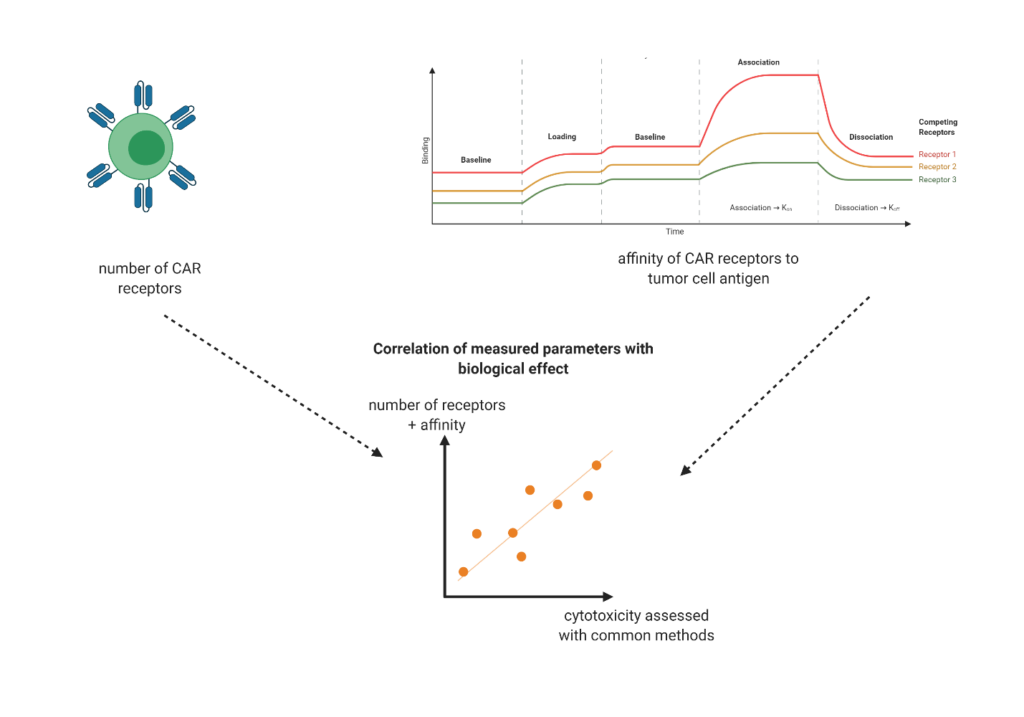
Multiple Technologies, Assays, and Hours Required
As mentioned above, CGT manufacturing involves testing of many parameters, be it molecular, biochemical, immunologic, phenotypic, physical, or biological. Testing all of these attributes requires many different technologies: qPCR for identity testing, ELISA for purity testing, various kits for contaminant analysis… Alone, these technologies are often relatively fast and simple to handle; however, needing to test all them in parallel with multiple different technologies becomes an increasingly difficult task. Furthermore, the diversity of the technologies, the expertise of the personnel, and the overall time and costs required to cover all of the bases in QC testing greatly hampers the scalability and outsourcing of CGT manufacturing.
Replacing all technologies or all biotools with a single one is a highly unlikely scenario, as the measurements required differ vastly from one another. However, reducing the number of technologies used in the workflow could greatly ease the difficulties in outsourcing of CGT manufacturing and upscaling production. Firstly, this would help reduce the costs of manufacturing by reducing both the number of technologies (and thereby also the respective consumables), and the trained personnel required to perform the assays. Secondly, assuming the technology in question has the ability to multiplex measurements, having one technology rather than many also greatly reduces the amount of time required to assess quality attributes. Technologies such as focal molography, capable of performing many of the routine assays undergone in QC, i.e. identity, purity, potency, and safety testing, are therefore highly desirable in this environment. Furthermore, automation of assays and technologies capable of being integrated into closed-loop control systems are also of great advantage, as they enable significant decrease of hands-on time and necessary technical expertise, features that Molography is also compatible with.
Re-purposed Technologies
One of the reasons so many platforms and technologies exist in the CGT workflow is that many of them stem from the “traditional” pharmaceutical industry, e.g. small molecule- and protein manufacturers, and were re-purposed for use in CGT manufacturing. One example of such a repurposed technology is the use of Thermo Fisher’s affinity gels, commonly used for monoclonal antibody purification, for AAV purification. Although the use of established technologies has been sufficiently successful thus far, we are currently reaching the limits of what we can achieve in CGT manufacturing with them, as only small modifications to the manufacturing workflow can be undertaken. Indeed, some of these technologies do not enable sterile enough workflows, or greatly complexify it to adhere to current CGT manufacturing standards. Others do not allow sensitive enough measurements to accurately read out desired parameters for CGTs, which in turn gives a too vague idea of the drug products’ effects in patients.
The need for technologies designed to accommodate the higher maintenance workflows demanded in CGT manufacturing is becoming increasingly clear. In particular, technologies capable of being integrated into closed manufacturing loops, and offering the possibility of automation, have significant advantages. They allow manufacturers to expand existing capacity and bringing the manufacturing of CGTs closer to or at the point of care. These changes in turn reduce costs because of the decrease in transportation and personnel required. The technological challenges faced by CGT manufacturers is one that lino aims to help solve with Focal Molography.
Outlook
Improvements in the CGT manufacturing workflow are becoming increasingly necessary; as the demand for CGTs grows, manufacturers must find ways to adapt existing systems to increase production whilst maintaining or even improving quality of products. Ultimately, by challenging the way our manufacturing systems are designed today, we not only help decrease time and costs for manufacturers, but more importantly, we can improve patient care by providing better access to innovative and breakthrough therapies.
References
Biorender.com
https://www.labroots.com/webinar/car-t-generation-identity-purity-potency-sterility-assay-testing
https://www.mckinsey.com/industries/life-sciences/our-insights/a-call-to-action-opportunities-and-challenges-for-cgts-in-europe
https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q05c_pf_ira_33_2_2007.pdf
https://www.fda.gov/media/79856/download
https://www.sartorius.hr/media/1rifb01r/qc-in-viral-vaccine-and-vector-production-brochure-en-l.pdf

